OUR FOCUS
At Cortalix we are developing an internal and growing pipeline of radiopharmaceuticals. Our internal programs largely consist of radiopharmaceuticals for fibrogenesis-related membrane receptors and proteins that occur in early-stage fibrotic diseases, such as pulmonary fibrosis, cardiac fibrosis and liver fibrosis, but also in various solid cancers with fibrotic stroma, such as colon cancer and pancreatic cancer.
OUR INTERNAL PROGRAMS
Our current internal programs include radiopharmaceuticals for the molecular targets PDGFRA (CD140a), PDGFRB (CD140b), EGFR, IGF-2R (CD222) and FAP. All our single domain antibodies contain a functional group that can chelate a radionuclide suitable for nuclear imaging (e.g. 68-Ga) or radiotherapy (e.g. 177-Lu).
This platform allows for a theranostic approach in cancer using the same singled domain antibody for patient identification, targeted therapy and disease monitoring (a combined diagnostic and therapeutic in 1 single domain antibody).
Our frontrunner CTX001 is a PDGFRB binding sdAb with excellent properties and is initially being developed as a 68-Gallium-labeled PET imaging diagnostic for idiopathic pulmonary fibrosis and colon cancer.
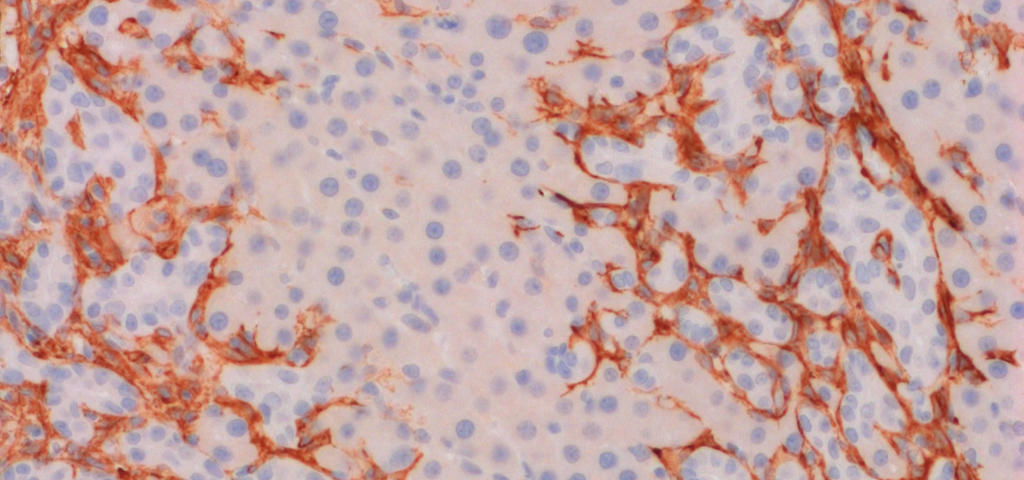
Fibrosis and fibrogenesis
Fibrosis is the consequence of chronic cycles of tissue injury and repair leading to the accumulation of scar tissue, mainly collagen, in affected organs and disruption of normal tissue architecture and function. Fibrosis is the final common pathway in virtually all forms of chronic organ failure. The cellular and molecular biology of fibrosis is similar across multiple organs and multiple sources of tissue injury, including fibrotic stroma in many solid cancers. Fibrotic stroma can make up to 80% of tumor mass and is a suitable target for cancer treatment.
Fibrogenesis is the mechanism of fibrosis and is reflecting fibrosis activity.
Why targeting fibrogenesis
Consequently, fibrogenesis is a far better target as it reflects fibrosis activity. Fibrogenesis is the mechanism of wound healing and repair. Upon scarring, there is migration, proliferation and differentiation of fibrogenic cells towards activated myofibroblasts, the key pathogenic cells, followed by deposition of extracellular matrix proteins, including collagen. PDGF and TGF-beta are the main drivers of fibrosis and are validated targets for fibrosis treatment. Activated myofibroblasts express specific types of membrane receptors that distinguish them from quiescent fibroblasts.
When targeting fibrogenesis, one targets the pathogenic cells of the active disease itself. Irrespective of the amounts of deposited collagen in the fibrotic organ and how fast it will disappear.
This is the area where Cortalix, as a fibrogenesis targeting company, has made fast progress in developing tracers for these specific membrane receptors, reflecting fibrosis activity.
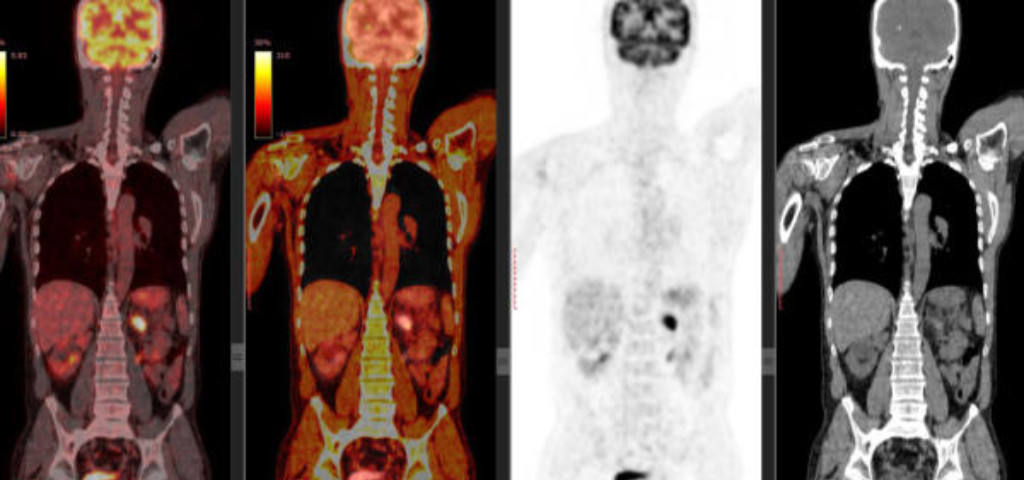
Diagnostic imaging of fibrogenesis
Diagnostic imaging of fibrosis is nowadays primarily focusing on major tissue density differences caused by scar tissue, mainly collagen. This could work for identification of patients with established fibrosis. But resolution of scar tissue after a potentially successful antifibrotic intervention may take many years. Therefore, todays’ diagnostic imaging of fibrosis is not suitable for screening and disease monitoring in patients. Moreover, lack of sensitive and reliable diagnostic options hamper the development of novel antifibrotic therapies. There is a growing demand for molecular imaging diagnostics allowing early-stage detection of fibrotic chronic diseases and help minimize costs of treating these diseases.
CTX001
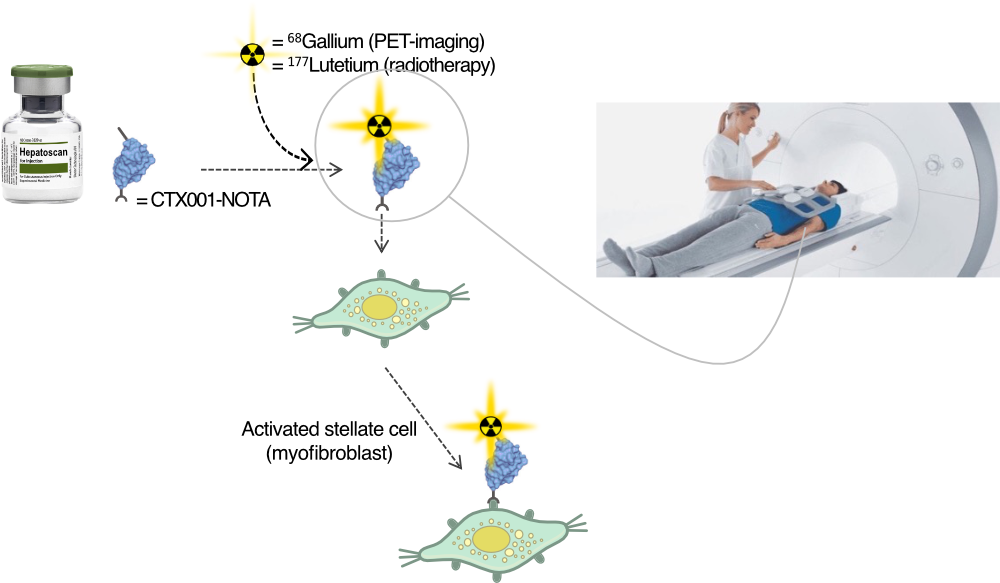
Cortalix’s lead product, CTX001, is based on a proprietary PDGFRB-binding single domain antibody library. PDGFRB (CD140b) is one of the first of a group of dynamic membrane receptors expressed on activated myofibroblasts, the main pathogenic cells causing fibrosis.
In the diagnostic imaging program 68Ga-NOTA-CTX001 (formerly BOT1712 from BiOrion Technologies), the compound chelates the PET radionuclide Gallium-68. The use of gallium has become popular in recent years with the development of Ge/Ga generators. We are also working on an 18-fluoro version of CTX001. The first human PET imaging studies with 68Ga-NOTA-CTX001 are expected in the first quarter of 2025.
If the PET imaging results and the calculated radiation burden on the human body permit, the development of CTX001 will be expanded to 177Lu-DOTA-MYR-CTX001 for ImmunoRadiotherapy in metastatic colorectal cancer.
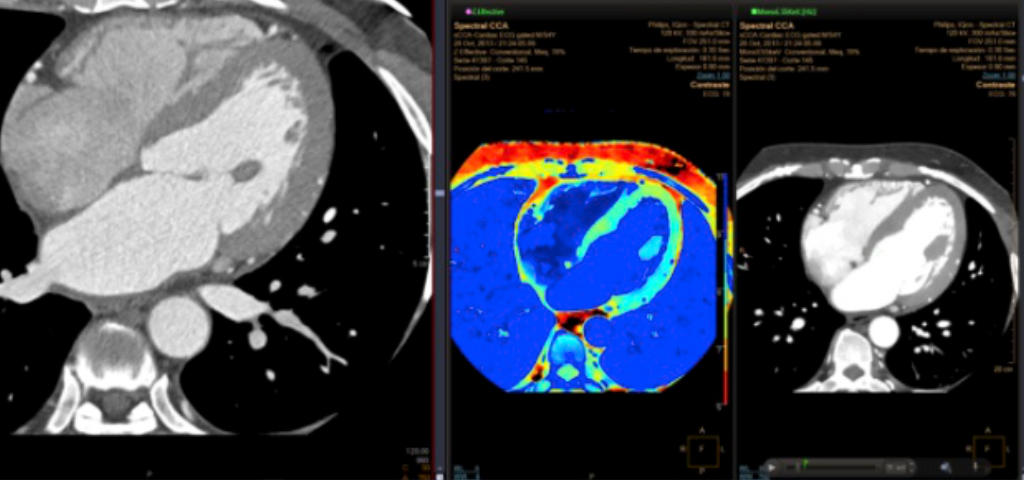
BOT5035 (thorascan)
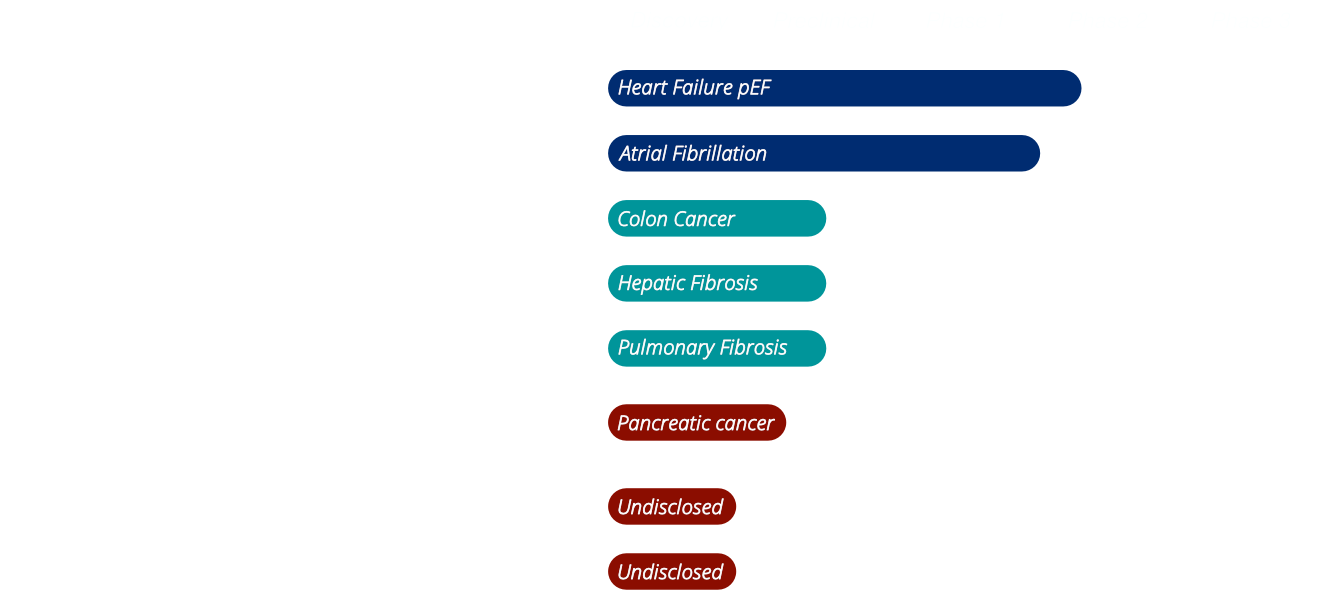
In the diagnostic imaging program 68Ga-BOT5035, Thorascan®, a first generation dimeric PDGF-ß-receptor binding bicyclic peptide is conjugated to NOTA, that chelates the PET radionuclide Gallium-68. In parallel, an 18-fluorine radionuclide is being developed. In addition to cardiac fibrosis (HFpEF and AFib), BOT5035 will be developed for diagnostic imaging of pulmonary fibrosis (ILD and IPF), allowing disease monitoring of current and future therapeutic strategies.
The BOT 5035 program was acquired from BiOrion Technologies in early 2023.
BOT5035 is so far tested in 2 clinical PET-imaging studies and a third clinical phase 2 PET-imaging study is anticipated for 2024
Other candidates
At Cortalix, we have selected additional candidates for novel diagnostic imaging tracers based on our single domain antibody discovery engine for PDGFRA, IGF-2R, EGFR and FAP.
CTX004, a PDGFRA binding single domain antibody, is being developed as the next pipeline product for imaging PDGFRA-positive pancreatic ductal adenocarcinoma (PDAC). These patients generally have a longer chance of survival due to greater susceptibility to immune cell infiltration. Targeted diagnostics on PDGFRA could contribute to identifying this patient group in order to provide appropriate treatment. No development indications have yet been chosen for the other diagnostic imaging tracers. These will be further developed towards leading programs in fibrosis and cancer.
