partner programs
Cortalix collaborates with other companies and academic institutions to select novel single domain antibodies for attractive targets. We stand out by understanding the characteristics of this field and we will continue to build on that in the future. We are experts in the selection of novel single domain antibody candidates from highly diverse libraries. Moreover, we can further improve and customize sdAbs through genetic engineering, chemical coupling (labeling), and adjust their kinetics using particular functional groups.
For the near future, we will develop additional services that make the step to clinical studies faster and less expensive. Producing a clinical GMP batch of several grams for an imaging diagnostic program is completely different from a conventional therapeutic trial that requires hundreds of grams. At Cortalix we invest in the development of cell banks for the production and ultimately the in-house production of small-scale GMP sdAb batches.
However, single domain antibody applications are much more versatile and extend far beyond just radiopharmaceutical applications. Therefore, we are eager to employ our libraries in collaboration with companies and research institutes that focus on other applications, such as neutraceuticals and neutralizing single domain antibodies or candidates for immunotherapy. We can also select sdAbs and use them to perform highly specific bioanalytical assays, such as ELISAs or protein purification procedures.
Projects that we do not carry out as a collaboration will be carried out as a service. See the services page for this.
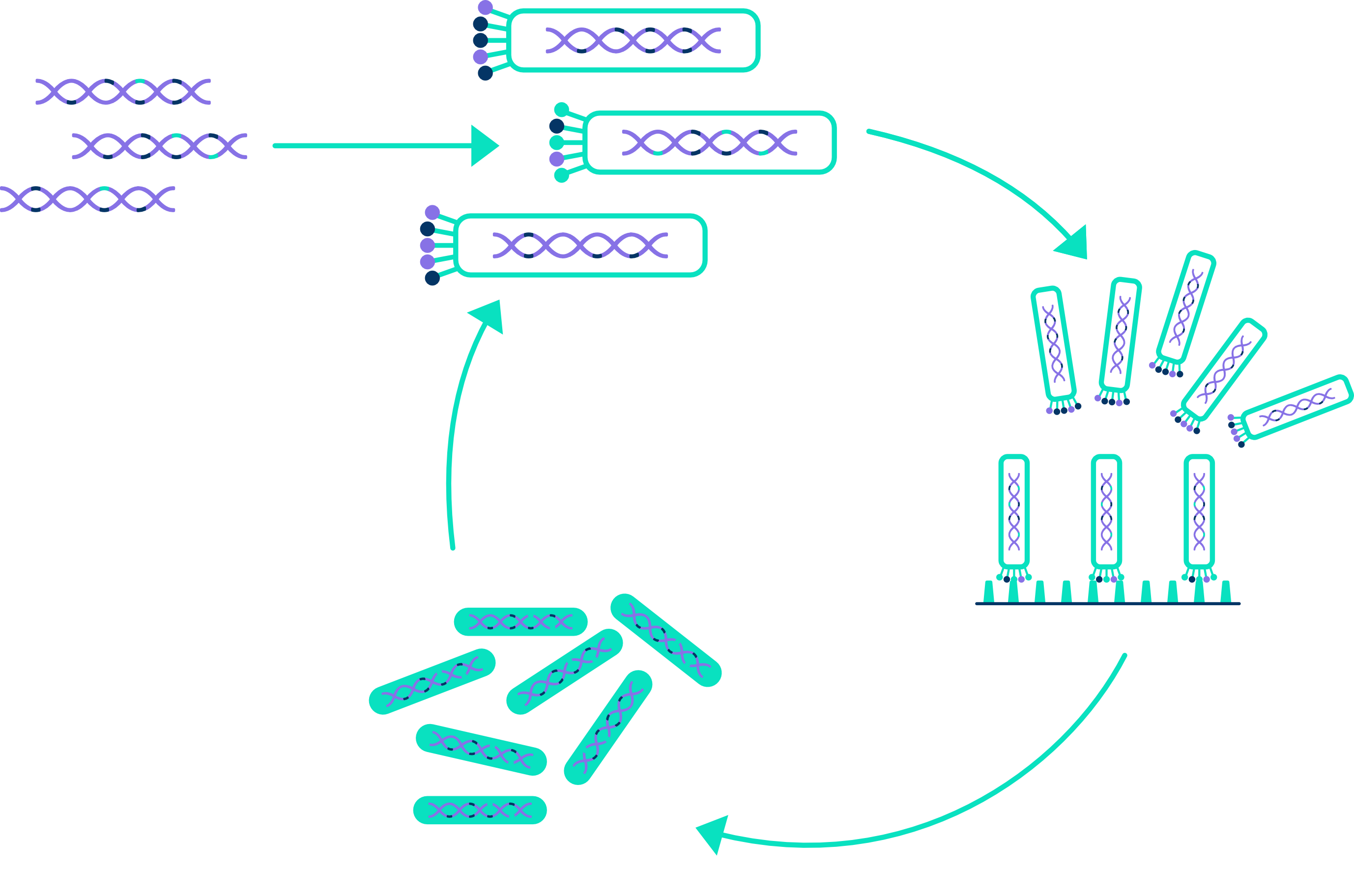
Phage Panning and Selection
With our collaborators, we will use our synthetic single domain antibody libraries to select candidate binders for a target protein of interest by using the phage display technology. Our synthetic single domain antibody libraries are based on generic robust scaffolds in which diversity was introduced through randomization of CDR3. An even more diverse library is obtained through an evolutionary process with regular mutations in the sdAb DNA sequence. Through biopanning, specific target binders are enriched using immobilized antigen or whole cells. After DNA sequence analysis of monoclonal binders, the sdAbs can be further characterized using various analytical techniques such as ELISA, SPR, immunofluorescence, and ultimately in vivo characterization/validation in disease models.
Expression and genetic engineering
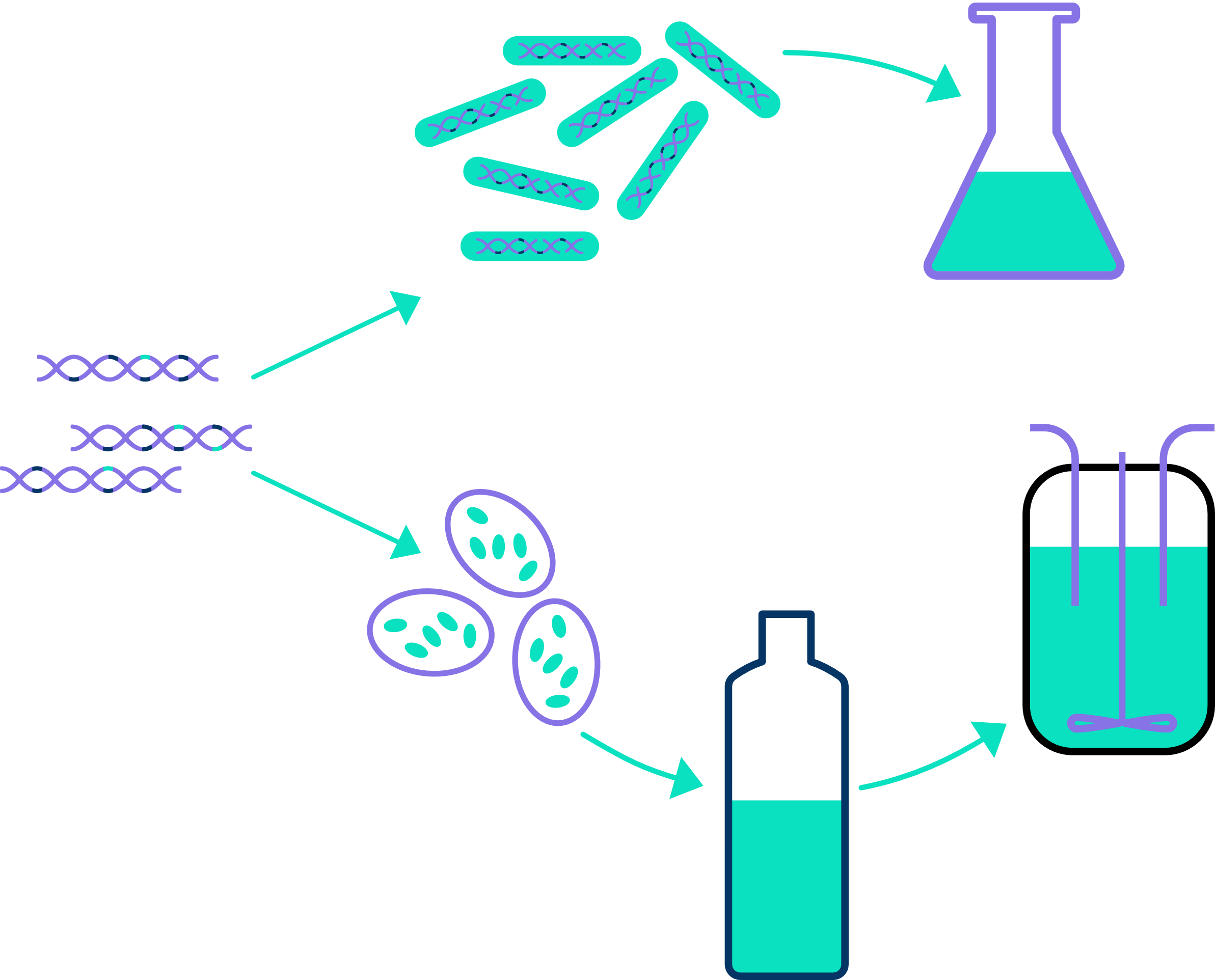
For the selected promising VHH-phage clones, we then construct expression vectors for single domain antibody production in bacteria and/or yeast, depending on the required quantity.
Small-scale production generally takes place in E. coli (0.1 – 1 mg) for early characterization, whereas for medium-scale production (1-25 mg) P. pastoris is employed for further in vitro and in vivo characterization in, for example, animal models.
A microbial cell bank can then be created for semi-large-scale production and pre-GMP technical batch optimization, up to 7 l fermentation.
Functionalization
We are experienced with the chemical coupling of sdAbs to a wide array of functional groups (fluorescent or NIR dyes, NOTA/DOTA/DTPA, etc.) to obtain a final sdAb conjugate fitting your application of interest. A customized linker can be designed for the conjugation of multiple functional groups to a sdAb, for example a long fatty acid to improve the PK profile.
We can also create bispecific and multi-specific sdAbs to target multiple targets simultaneously. Furthermore, humanization or a liability analysis can be performed. However, this is often not necessary for imaging diagnostics, which are often used at very low concentrations and only once or at most a few times. .
For the characterization of final single domain antibody candidates, cell lines with an inducible expression system for the antigen of interest can be used for cell-based biopanning and
FACS or SPR analysis.
