Custom VHH Antibody Development Services
At Cortalix, we provide advanced VHH antibody development services tailored to your needs, from animal-free synthetic libraries to llama-based immune libraries, and from binder selection to engineering and expression.
1. Choose Your Starting Point
a. Synthetic Library Discovery
Ideal for rapid screening, high-throughput projects, modular applications, or when animal immunization is not preferred.
- Animal-free
- Immediate start
- Broad binder diversity
- Compatible with multiple target classes (proteins, peptides, toxins, etc.)
b. Immune Library Discovery
For projects that require high-affinity binders, deep epitope coverage, or involve difficult targets, we offer the development of immune VHH antibody libraries based on llama or alpaca immunization with your antigen.
The immunization itself is carried out by one of our trusted specialized partners, who operate professional llama and alpaca facilities. Cortalix coordinates the process closely to ensure scientific alignment, quality, and timelines.
- Target-specific immune response
- High natural affinity
- Deep epitope coverage
- Immunization coordinated by Cortalix, performed at a certified facility
- Library construction, phage display and clone selection in-house at Cortalix
2. Selection & Enrichment (both routes)
Once your library (synthetic or immune) is in place, we proceed with tailored phage display panning to enrich for target-specific VHHs.
We optimize each round for stringency, specificity, and diversity, based on your application (diagnostic, therapeutic, etc.).
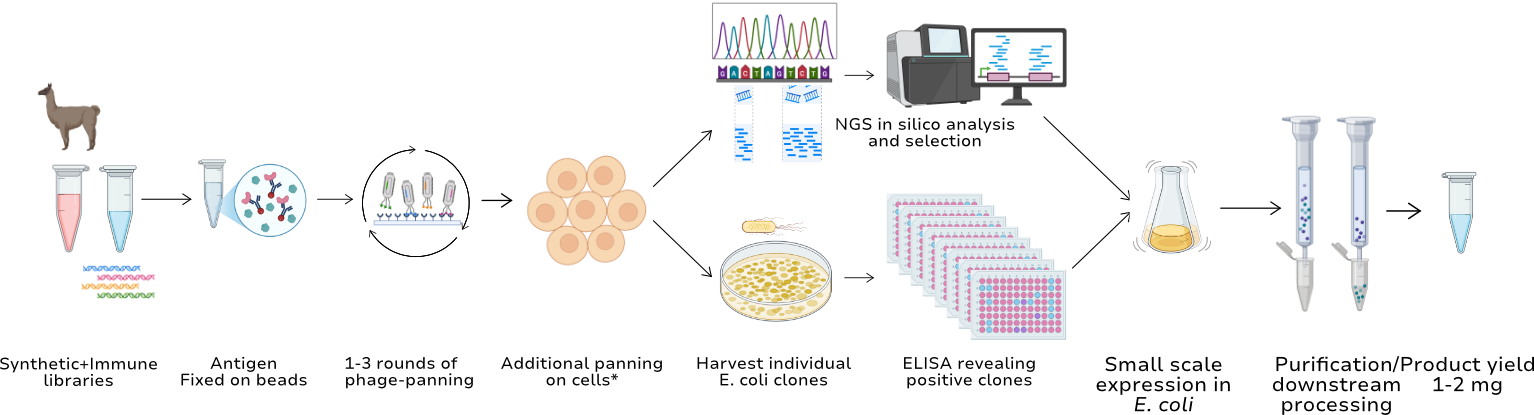
3. Clone Identification & Validation
From enriched pools, we isolate individual clones and characterize them via:
- ELISA
Optionally, we can provide high-throughput screening of 96+ clones.
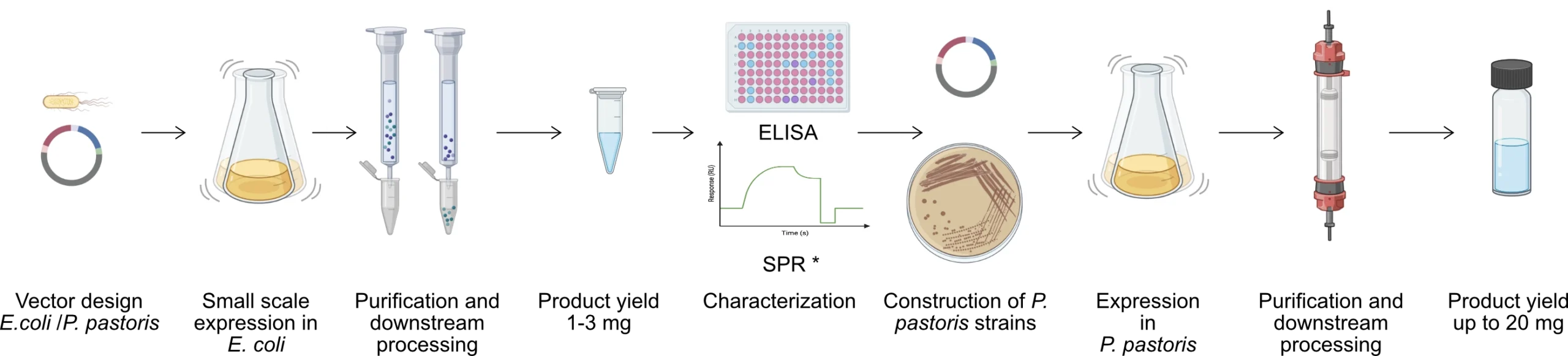
4. Downstream Engineering & Optimization
We offer additional services to tailor VHH Nanobodies to your needs:
- CDR grafting
-
Humanization
-
Affinity maturation
-
Fc-fusion or multimerization
-
Biotinylation or other conjugations
5. Expression & Scale-up
Expression of selected VHH antibodies in E. coli, Pichia pastoris, or mammalian systems.
- Small-scale expression & purification
- Scalable production-ready formats
- Endotoxin-free options available for in vivo use
6. Optional Add-ons
-
Diagnostic assay integration (e.g., ELISA or lateral flow)
- Functional groups and linker conjugation
-
Fluorophore or Chelator labeling for imaging or therapy
- Coupling to beads for analytical purposes
-
Custom formatting for analytical platforms (e.g., biosensors)
VHH Antibody Development Services: Flexibilty by Design
Our hybrid discovery model, offering both synthetic and immune VHH libraries, allows us to adapt to your project’s unique needs. Whether you require speed and modularity, or maximum affinity and epitope coverage, we help you choose the best route without compromise.
Every step is fully modular, so you can engage us from early discovery to downstream formatting, or anywhere in between.
Let’s Discuss Your Project
Not sure whether to choose synthetic or immune from our VHH antibody development services? We’re happy to advise based on your target and goals.
Contact us for a free consultation.
Frequently Asked Questions
Do you work with both synthetic and immune libraries?
Yes. We offer both synthetic libraries (ready-to-screen) and immune libraries based on llama or alpaca immunization with your antigen.
Can I supply my own immunized material?
Yes, if you already have cDNA from your isolated PBMCs or RNA from immunized animals, we can take it from there, clone it into a suitable phage vector, and build the immune library.
How unique are the VHHs that Cortalix delivers?
The immune libraries that Cortalix obtains are highly diverse, and the likelihood of two customers receiving the same VHH sequence is extremely small. We make every effort to ensure that each clone delivered is unique. In cases where different customers request VHHs against the same target, we take strict measures to avoid assigning the same sequence more than once.
Do we have full ownership of the delivered VHH sequences ?
Yes, in almost all cases you receive full ownership of the nanobody DNA and amino acid sequences that originate from immunizations performed on your behalf. When using our proprietary synthetic libraries, which include specially developed frameworks or patented sequence elements (e.g., in spacers or linkers), we can grant you a non-exclusive or, under certain conditions, an exclusive license for worldwide commercial use. This provides the assurance that you can freely use and further develop the VHH antibodies, while benefiting from the unique properties of our proprietary designs.
Can we request only selection services?
Yes, that is indeed a very logical step to start with.
Do keep in mind, though, that for any of the later development stages it is often necessary to revisit some of the preceding steps. For example, to move forward we may need to reclone the VHH into a different backbone, express it in a suitable system, or engineer it with specific linkers and functional groups to ensure functionality in the intended application. These are standard parts of the workflow and help us secure robust performance and scalability.
Just let us know what your intended use is, and we will map out the options together. Our aim is to keep the process as efficient as possible, while making sure that each nanobody is engineered and validated in a way that supports your downstream goals.
What is the typical timeline?
Synthetic library projects can start immediately and deliver binders in 4–5 weeks. Immune library projects depend on immunization timelines (typically 6-8 weeks) followed by discovery.
Do we only get the amino acid sequence at the end of STEP 1 or also the protein?
No. For VHHs obtained from immune libraries, we can, if desired, provide the DNA sequence of the VHH itself. The amino acid (and DNA) sequence of any spacer, linker, or proprietary framework is not disclosed, as these are part of additional services (Step 5 and/or 6). Where proprietary spacers and/or linkers are used, a non-exclusive or exclusive license can be granted for their worldwide use.
Do you guarantee a number of unique VHH binders from your library?
Yes, if we accept the target you supply (e.g., sufficiently pure, large enough, and suitable for immobilization (biotin) on magnetic beads or a 96-well plate), we commit to a best-effort selection process. Our goal is to deliver at least two unique binders. For most targets this is realistic, but please note that 100% guarantees cannot be given, some proteins or epitopes are inherently difficult. If the first selection round does not yield two unique binders, we will repeat the process free of charge. Should this still result in fewer than two binders, we will only charge 50% of the agreed price for this step.
What is the quantity and quality of the expressed VHH from step 2?
For initial characterization, a small batch of VHH is usually sufficient (≈1-2 mg). At this stage, the nanobody is cloned into a production strain of E. coli, expressed, and purified through multi-step chromatography, yielding >95% purity. This allows us to assess affinity, specificity, and selectivity. By default, we produce the smallest batches in E. coli; for larger quantities or specific applications, production can be scaled up in Pichia pastoris or adapted to alternative systems.
Which functional groups do you conjugate?
We have extensive experience in conjugating functional groups to VHHs, with a strong focus on C-terminal cysteine conjugation. This is our preferred strategy because it delivers high coupling efficiency while maintaining VHH binding activity. Using maleimide chemistry, we routinely attach labels such as sCy7, NIR-800-CW, NOTA, DOTA, DTPA, and long-chain fatty acids. To minimize the risk of conjugation interfering with target recognition, we avoid introducing unnecessary cysteines in both the CDRs and the framework regions. The resulting conjugates typically achieve >95% purity, confirmed by LC-MS analysis.
It should be noted, however, that conjugation can sometimes alter the affinity for a given epitope, especially when the precise details of the VHH–epitope interaction are not fully understood.
