Lateral Flow Assay
What is a Lateral Flow Assay
A lateral flow assay (LFA) is a rapid, often low-cost diagnostic test that resembles an ELISA but runs on a paper strip without lab equipment.
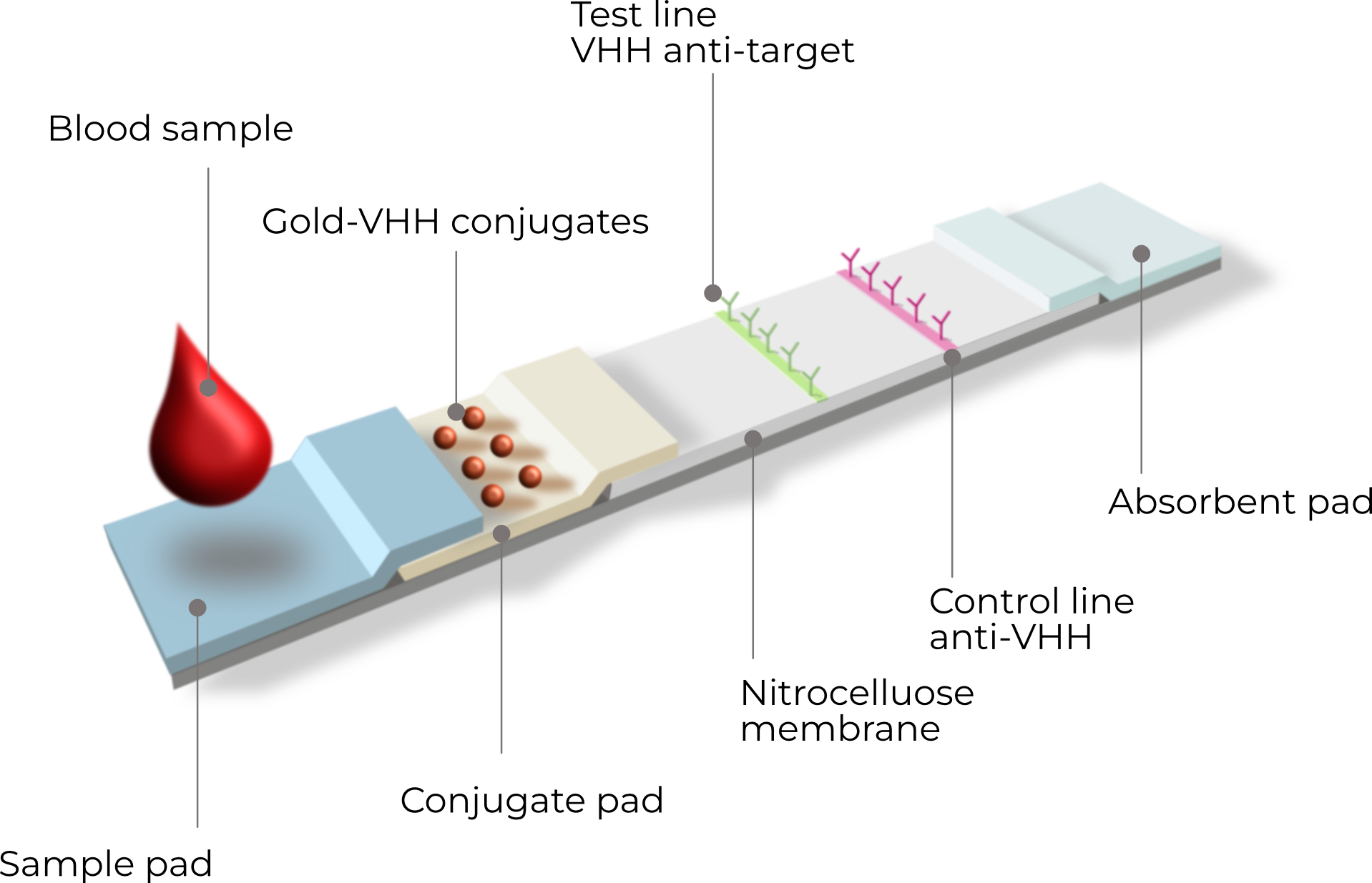
Most people remember LFAs from the COVID-19 self-tests many of us used. The strip has a sample pad, conjugate pad (with labeled binders, e.g., gold-nanoparticle antibodies/VHH antibodies), nitrocellulose with test and control lines, and an absorbent pad.
You add a sample (blood/serum/saliva/urine/buffer); capillary flow carries it along, forming target–label complexes.
At the test line, immobilized binders capture those complexes to produce a visible band; the control line confirms proper flow and reagent integrity.
Like ELISA, LFA uses specific binders, but the readout is fast (≈10–20 min) and instrument-free at point-of-care.
Two main formats: sandwich (proteins/viruses) and competitive (small molecules).
ELISA is typically more sensitive/quantitative; LFAs trade some sensitivity for speed, simplicity, and cost.
While LFAs have traditionally used conventional antibodies, the rise of antibody fragments, such as VHH antibodies, opens the door to binders that can be better, faster to produce, more cost-effective, and more stable.
VHH-Antibodies for Next-Gen LFAs
VHH antibodies are compact, highly stable, and easy to express in E. coli. and Pichia pastoris. That makes them ideal reagents for LFA rapid tests, where consistent performance and supply certainty are crucial.
Why VHH Antibodies in LFAs?
-
High stability & small footprint → supports high binder density and clean signal formation on nitrocellulose.
-
Fast, scalable production in bacterial or yeast expression reduces cost and lead time.
-
Excellent specificity → in general, smaller epitope recognition allows selection of unique binders without cross-reactivity against comparable targets.
How we approach VHH antibody LFAs
- Matched pairs for sandwich formats
We provide high-affinity VHH binders from our immune and synthetic libraries, and can identify non-competing pairs that are suitable for capture and detection roles in an LFA.
- Collaboration on assay architecture
Critical parameters for conjugation chemistry, pad treatments, blocking buffers and strip layout are highly assay-specific. Here, we work closely with our partners: you define the intended use case and performance requirements, and we contribute our nanobody expertise plus initial feasibility testing.
- Tagging considerations
Some protein tags can interfere with assay performance. Where relevant, we take these aspects into account during discovery and cloning, to help avoid artifacts later in the workflow.
What we deliver
-
VHH antibody discovery & engineering: From target definition to matched binder pairs, using immune and synthetic libraries.
-
Feasibility data: Initial tests of binder pairing, competition, and expression suitability for LFA applications.
-
Collaboration on assay development: Together with your team, we design the conjugation, pad/buffer system and strip layout.
-
Stability & specificity assessment: Where possible, we evaluate stability and cross-reactivity, but final validation depends on the intended use case and matrix information provided by our partners.
Use cases & outlook
Our role is to supply and tailor VHH binders as the key reagents for LFAs. The exact sensitivity, matrix compatibility, and strip performance will depend on assay design choices that are best made in close collaboration with you. Together we can take VHH-based LFAs from concept to validated prototype.
Select Source
- Peng, Y. et al. Nanobody-Based Lateral Flow Assay for Rapid Zika Virus Detection, ACS Synthetic Biology (2025), 14-890-900.
- Parolo, C. et al. Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays, Nature Protocols (2020): doi.org/10.1038/s41596-020-0357-x.
