MultiDomeTM Platform
MultiDome™ – Multivalent and Multispecific VHH Antibody Platform
What is MultiDome™
MultiDome™ is our proprietary platform for designing multivalent and multispecific VHH antibody constructs. By linking two, three, or more VHHs with carefully selected linkers, either flexible or more rigid, we create constructs that maintain all the inherent advantages of monovalent VHHs, while offering entirely new functional opportunities.
Unlike scFv fragments, which often retain hydrophobic patches that make them unstable and difficult to express, MultiDome™ constructs are highly soluble, stable, and easy to produce. This makes them a superior choice for therapeutic and diagnostic applications where stability and scalability are critical.
How it works
MultiDome™ allows the modular assembly of VHH antibody domains in different architectures:
-
Bi- or tri-specific constructs: simultaneously targeting two or three different proteins.
-
Multi-epitope constructs: recognizing distinct epitopes on the same protein, enhancing avidity and functional activity.
-
Valency tuning: engaging the same epitope on multiple molecules of a target protein to boost binding strength.
The choice of linker, flexible or rigid, short or extended, determines how the VHH domains interact structurally and functionally. This flexibility allows for optimal construct design tailored to the biology of the target.
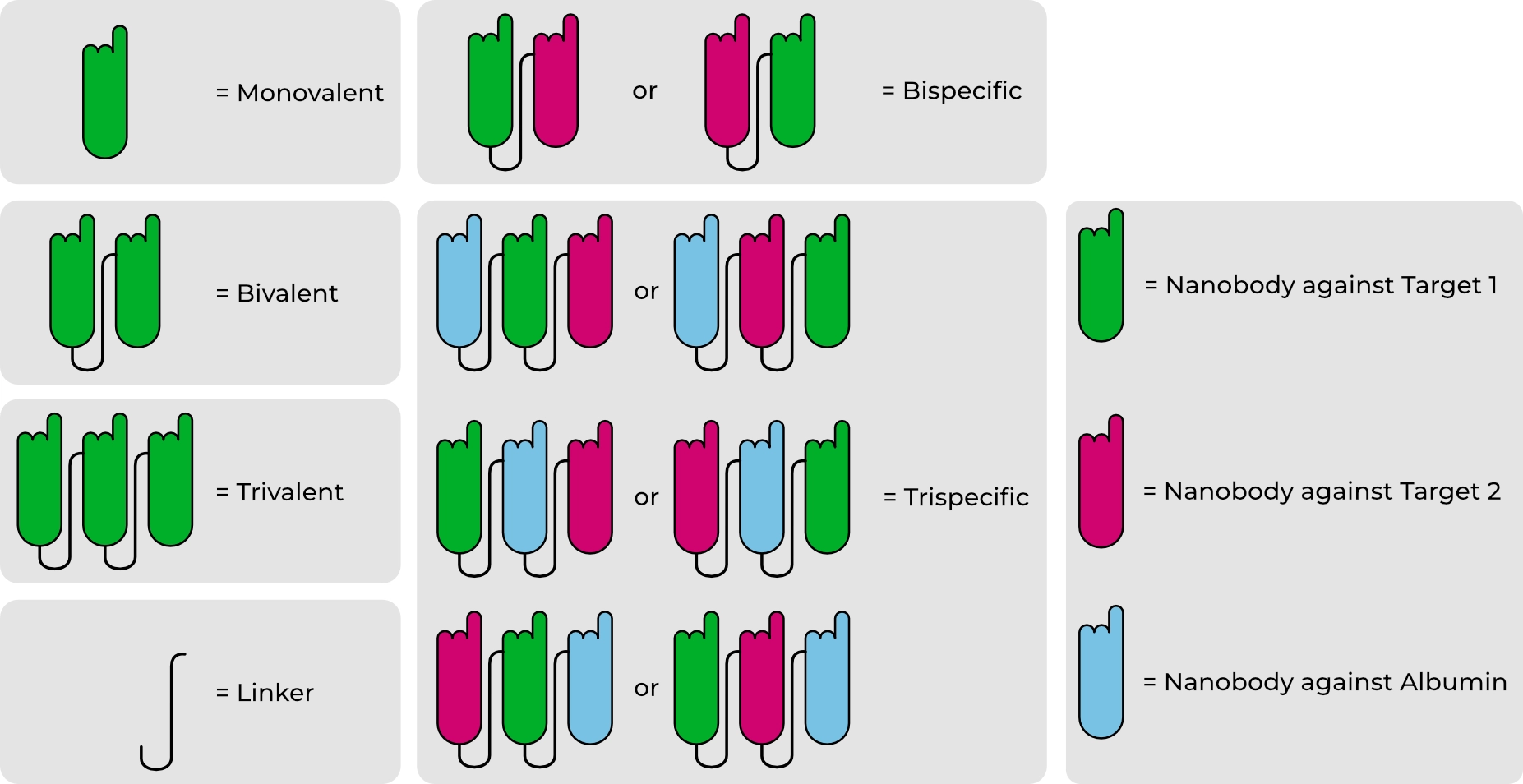
Figure: Examples of multivalent and multispecific VHH antibody constructs. Individual VHH domains can be linked flexibly or rigidly to form bi- or tri-valent molecules, or combined to generate bispecific and trispecific formats. Target-binding VHH antibodies (green, pink) can be combined with albumin-binding VHH antibodies (blue) to optimize pharmacokinetics while retaining the inherent stability and solubility of monovalent VHH antibodies.
Framework quality
Every VHH antibody scaffold within the MultiDome™ platform has been thoroughly optimized. Our frameworks are >90% humanized, minimizing the risk of immunogenicity. In addition, they are free of liability sites that could otherwise result in unwanted post-translational modifications. This ensures stable, safe, and translationally relevant constructs for therapeutic development.
Advantages of MultiDome™
- Retains all benefits of monovalent VHH antibodies: small size, high stability, easy expression.
- Superior stability compared to scFv or Fab fragments.
- Modular design with flexible or rigid linkers for tailored geometry.
- Humanized, liability-free scaffolds for low immunogenicity.
- Applicable to oncology, immunotherapy, and radiopharmaceuticals
Applications
-
Oncology: dual targeting of tumor antigens to improve efficacy.
-
Radiopharmaceuticals: tri-specific tracers combining two cancer targets with an albumin-binding domain from our AlbuFlex™ platform, for optimized PK.
-
Inflammation and fibrosis: multi-epitope constructs to increase potency and selectivity.
Our approach
At Cortalix, we systematically design and test MultiDome™ architectures, adjusting valency, epitope targeting, and linker flexibility. In collaboration with partners, we develop constructs that translate into more effective therapies and diagnostics.
Select Source
-
Jovčevska I, Muyldermans S. The Therapeutic Potential of Nanobodies. BioDrugs. 2020;34:11-26. doi:10.1007/s40259-019-00392-8.
-
Steeland S, Vandenbroucke RE, Libert C. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today. 2016;21(7):1076-1113. doi:10.1016/j.drudis.2016.04.003.
-
Van Audenhove I, Gettemans J. Nanobodies as Versatile Tools in Inflammation Research and Therapy. Front Immunol. 2016;7:407. doi:10.3389/fimmu.2016.00407.
-
Harmsen MM, Ackerschott B, de Smit H, et al. Serum immunoglobulin or albumin binding single-domain antibodies that enable tailored half-life extension of biologics in multiple animal species. Front Immunol. 2024;15:1346328. doi:10.3389/fimmu.2024.1346328.
-
Van Lith SAM, et al. Novel VHH-Based Tracers with Variable Plasma Half-Lives for Imaging. Mol Pharmaceut. 2022;19(7):2192-2205. doi:10.1021/acs.molpharmaceut.1c00841.
