Our VHH Antibody Discovery Platform
Flexible by Design. Powerful in Execution.
At Cortalix, we built our VHH antibody discovery platform around one simple principle: no single discovery route fits every target. That’s why we offer two fully integrated, complementary approaches for VHH discovery:
- Synthetic libraries for speed, modularity, and animal-free selection
- Immune libraries for target-specific binders with natural affinity maturation
You can choose either route, or combine them, we help you make that decision based on target complexity, timeline, and goals.
Our Discovery Libraries
At Cortalix, we initially created immune libraries from llamas immunized with a mixture consisting of extracellular domains of membrane receptors overexpressed in fibrosis and various cell types present in fibrotic tissue and fibrotic cancers.
Starting from these immune VHH libraries, we were able to select a large number of attractive VHH candidates which can be developed into clinical candidates after various in vitro and in vivo studies.
Based on our previous experiences with immune libraries, we have invested in the design and construction of animal-free synthetic sdAb libraries.
For this purpose, we selected robust VHH frameworks in which the antigen-binding regions in the three CDRs were (partially) randomized using trimeric phosphoramidites to avoid, for example, unwanted stop codons or e.g. cysteines.
Synthetic Library Platform
Our synthetic VHH antibody libraries are designed for:
- High diversity (>10⁹
unique clones) - Rapid screening (within days)
- Modular design for engineering flexibility
- Fully animal-free discovery workflows
Synthetic panning allows you to quickly identify VHH antibodies with favorable properties, e.g. for targets that are toxic, or difficult to immunize, e.g. GPCRs. It’s ideal for diagnostic or time-critical applications, or when ethical or regulatory constraints limit animal use.
Immune Library Platform
For complex or highly specific targets, our immune library route is the preferred choice. We immunize llamas with your antigen of interest and generate VHH antibody libraries from the B-cell response.
Benefits include:
- Naturally high-affinity binders
- Conformational epitope recognition
- Matured specificity through the immune system
Immune panning is ideal for targets such as GPCRs, ion channels, or low-abundance proteins.
Shared Engineering & Optimization Layer
Regardless of the discovery route, all projects benefit from our advanced VHH antibody engineering capabilities:
- CDR grafting & humanization for therapeutic readiness
- Affinity maturation using rational design or mini synthetic library display systems
- Stability engineering for harsh environments (e.g., in vitro diagnostics)
- Conjugation with fluorophores, chelators, toxins, other drugs, Fc domains or enzymes
These tools allow us to optimize any lead for downstream use — in the lab, the clinic, or in commercial applications.
VHH Antibody Engineering & Optimization
Humanization
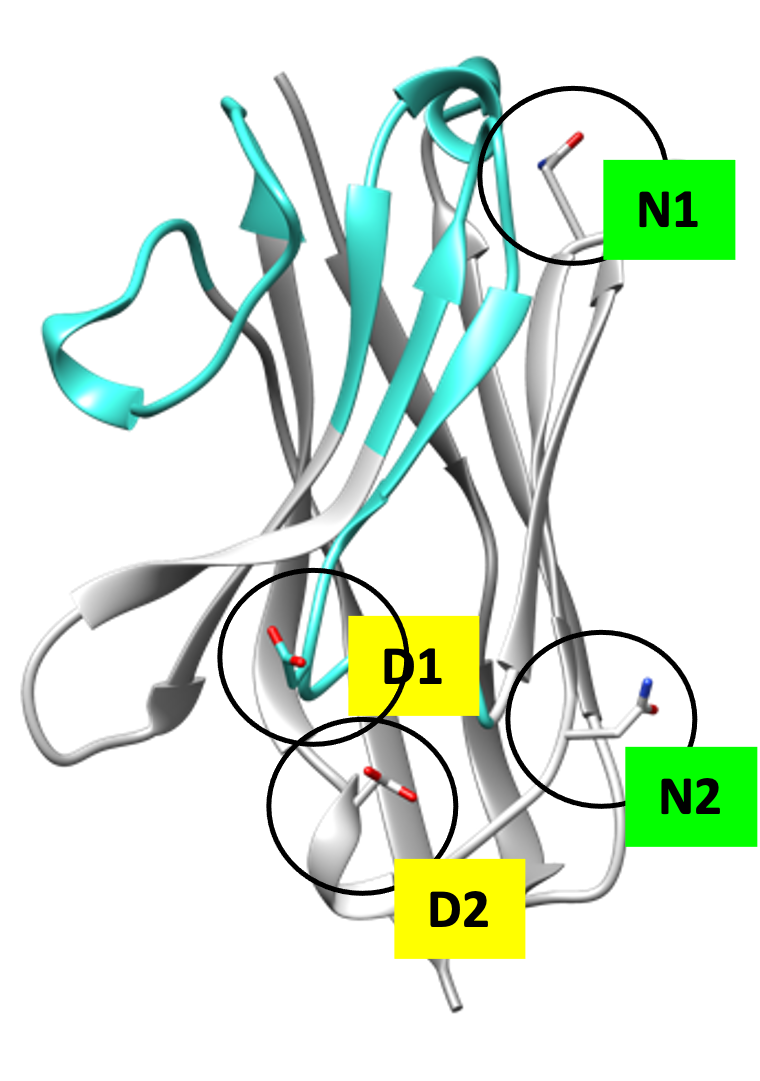
It is actually not possible to make a 100% humanized VHH antibody as that would disrupt the integrity and therefore the functionality of the VHH antibody. It is possible to approximate that situation as best as possible by replacing some amino acids of the framework, applying residue optimization, CDR grafting and finally evaluating the potential immunogenicity assessment with in silico prediction tools.
By following these activities, we ultimately end up with synthetic antibody libraries with an optimized framework and variable CDRs that produce molecules with limited immunogenicity, while retaining their antigen-binding properties and good expression properties.
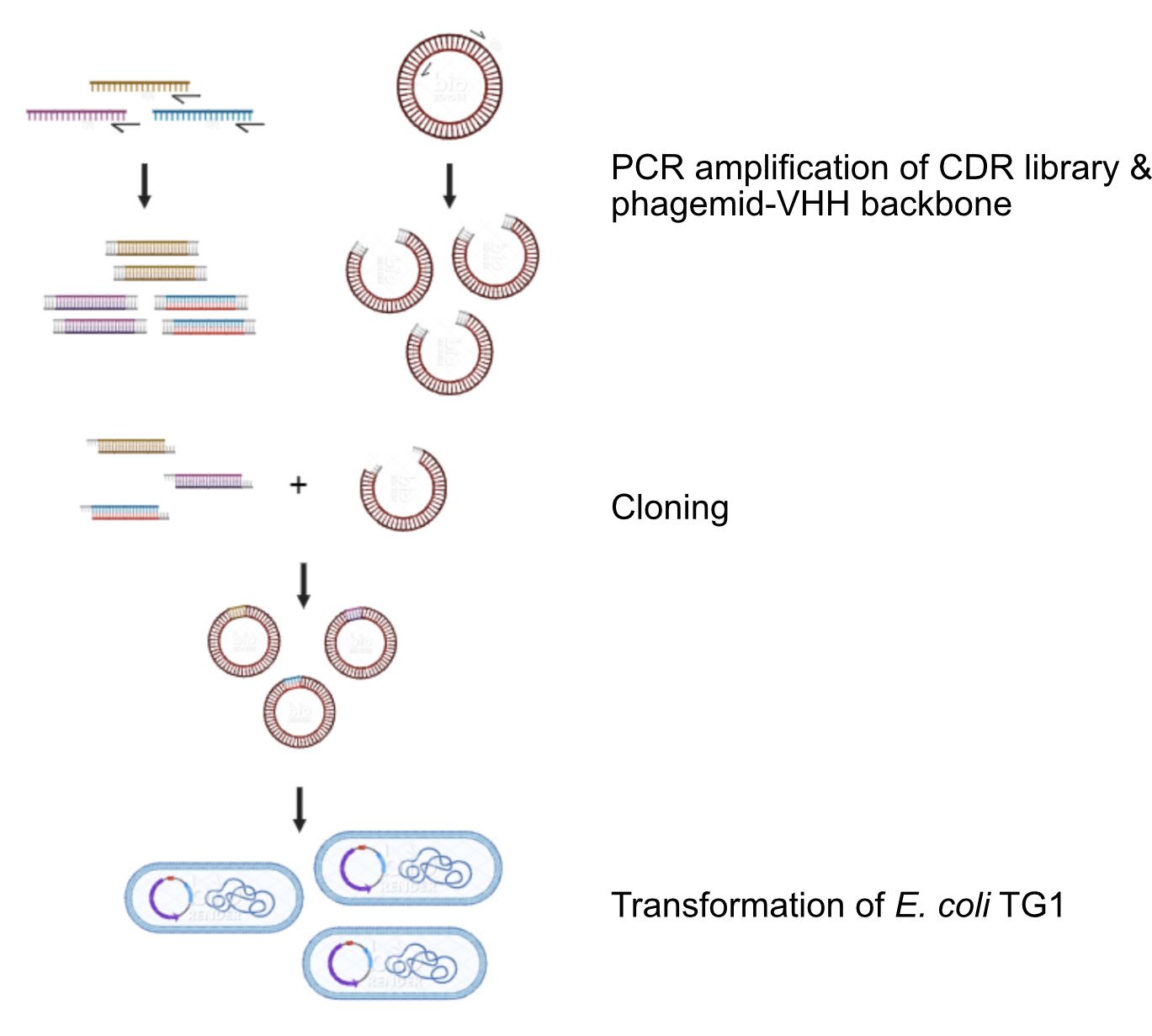
Cloning & Engineering
In the early days, when recombinant antibody technology, mainly focused on scFvs, emerged, VHH antibodies did not attract much attention. However, over the years, a steadily growing number of publications illustrated the benefits of VHH antibodies in certain research niches.
From Library to Tailored VHH
To identify practically applicable VHH antibodies, it is important to start from a high-quality gene bank of VHH antibodies, focused on the final application and with a great diversity. Our screening and selection procedure using phage display techonology allows us to extract the most promising VHHs for a particular target from this diverse library. Then, various options for genetic engineering and post-translational modification, including specific spacers and linkers, can be used to tailor the VHHs for its final application. Cortalix is experienced in the conjugation of VHHs to functional groups such as radionuclide chelators, including NOTA and DOTA derivatives or agarose beads. We use the latest techniques to control the PK and plasma clearance, which is different for a diagnostic and a therapeutic application/VHH antibodies.
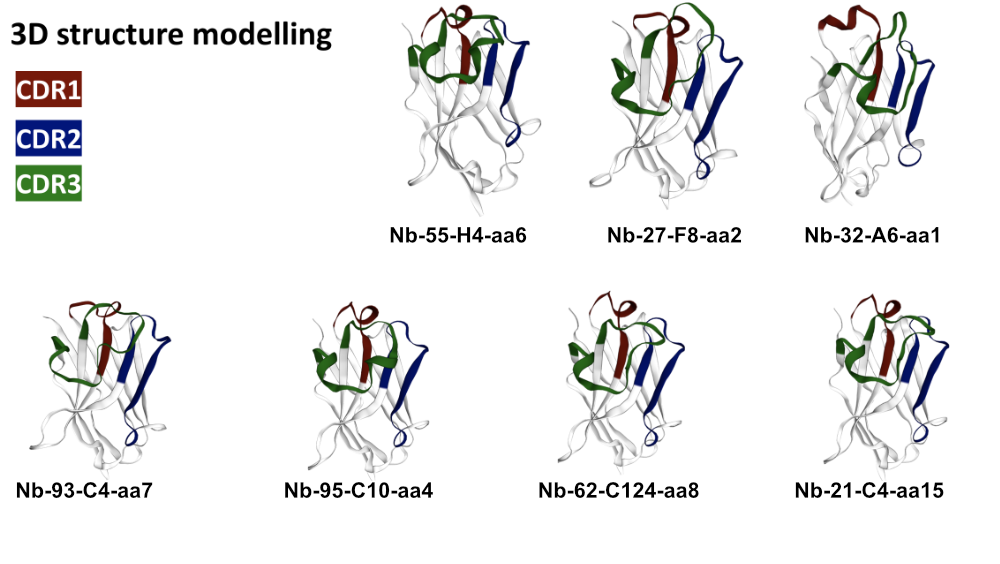
3D Modelling
Through 3D simulation we are able to make predictions, within certain limits, about the applicability of selected VHH antibodies.
Now that the first therapeutic VHH antibodies have been admitted to the market, other pharmaceutical companies will quickly join in and this will certainly lead to even broader applications, including in the field of radiopharmaceuticals and immunotherapeutics.
At Cortalix we want to participate in this development, and we will evolve into a party that will accelerate the application of VHH antibodiess. Cortalix aims to present solutions that offer prospects for patients who currently do not have them.
Flexible Routes, Integrated Output
You can start with a synthetic library for speed and parallelize with immune panning later, or vice versa. We adapt the discovery strategy to your target, not the other way around.
All leads, from either route, feed into the same downstream pipeline for characterization, optimization, and production preparation.
Let’s Talk
Let’s contact how we can introduce our VHH antibody discovery platform technology to support your antibody discovery program.
